Deliver Clinical Trial Transparency and Auditability
Pharmaceutical data and application workloads need higher levels of federated data protection and application access control. SafeLiShare helps govern app access and data protection in fully encrypted execution environments.
Automated Data Sharing Policy Compliance
Complete Privacy Audit of Data Availability
Run your data and application workloads with complete privacy, residency, sovereignty and transparent compliance. Encrypt all data in use, transit and at rest.
Balancing the Ease of Access to Shared Data Securely
A compliant trusted execution environment or TEE is is considered FIPS 140-2 level 3 compliant. It’s ideal for uni-party and multi-party confidential data access.
Safeguard Participant Anonymity
SafeLiShare extends PKI to prevent algorithmic attacks and unauthorized access without any proprietary PKI extensions.
Facilitate Immutable and Valid Research Output
Auditability and access control including automatic privacy compliance of data assets can be greatly simplified and automated.

“SafeLiShare is a technical implementation toward compliance. The realization of value was almost immediate - encryption from start to finish without de-identification going on in the secure enclave that saves the lengthy and labor intensive data prepping and masking prior to sharing.”
Vasanth Thangaraju, Vice President, Product & Technology
RxLive
Eliminate Algorithmic Attacks
By preventing algorithmic attacks and providing identity management to algorithm objects (apps), SafeLiShare enables large scale uni-party or multi-party data sharing with confidential data lifecycle enforcement.
Cloud Native Technology Infrastructure
Data deidentification and the potential for unauthorized reidentification have prevented some from considering sharing and even collaborating. SafeLiShare provides cloud-native agility and state of art encryption and policy enforcement technology to help alleviate the concerns.
Remote Attestation of Data Accuracy, Lineage and Visibility
- Tamper-proof data access audit on share data and apps with all parties access compliance
- Improve clinical trail data sharing and output access
People also ask
How does confidential computing improve clinical trials and drug development process?
What are some common regulations that Pharmaceutical companies must follow?
Does SafeLiShare provide capabilities like tokenization and data masking helps to secure financial PII? Why not?
Additional materials
February 21, 2024
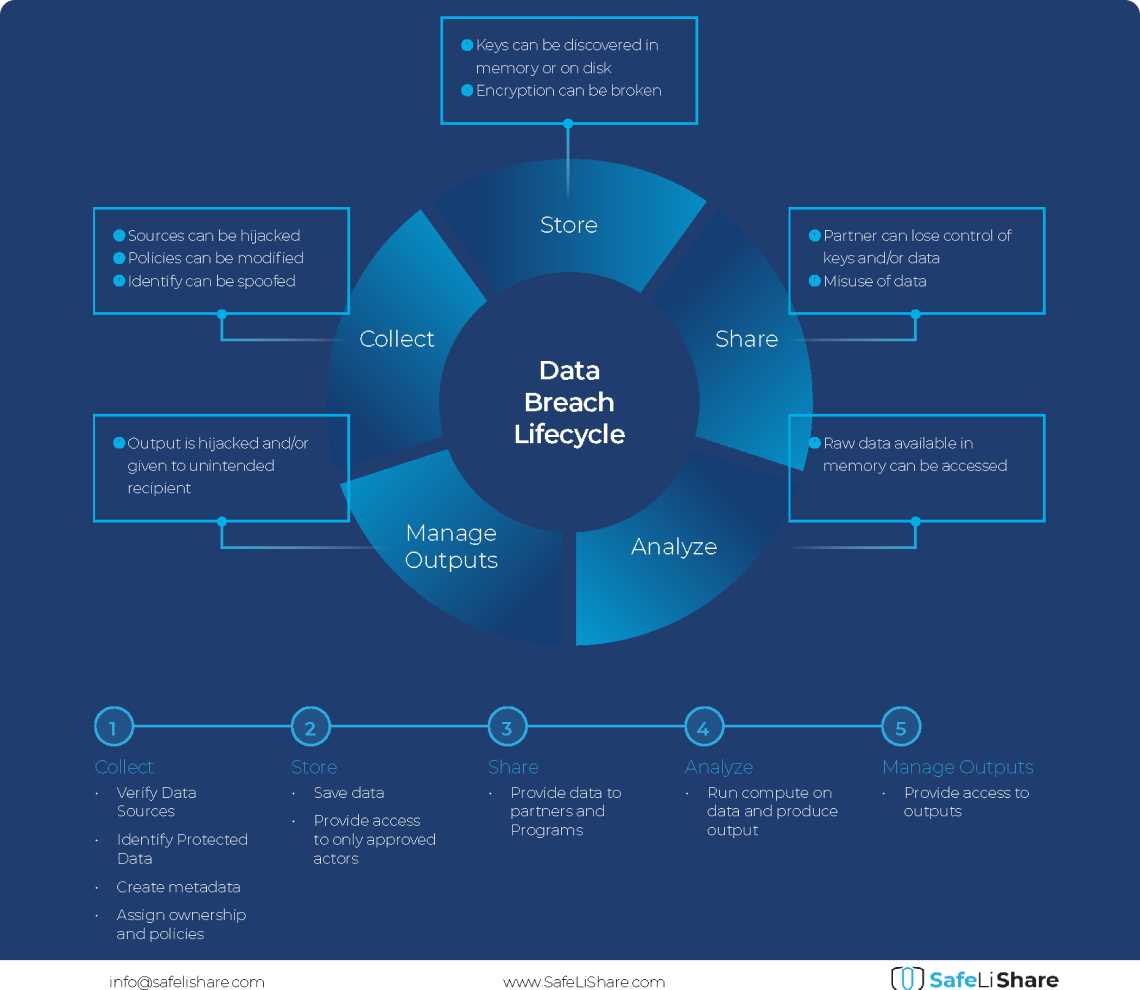
Cloud Data Breach Lifecycle Explained
During the data life cycle, sensitive information may be exposed to vulnerabilities in transfer, storage, and processing activities.

February 21, 2024
Bring Compute to Data
Predicting cloud data egress costs can be a daunting task, often leading to unexpected expenses post-collaboration and inference.

February 21, 2024
Zero Trust and LLM: Better Together
Cloud analytics inference and Zero Trust security principles are synergistic components that significantly enhance data-driven decision-making and cybersecurity resilience.
Experience secure collaborative data sharing today.
Maximize accessibility and monetization of sensitive, regulated, or confidential data without compromise.